Certified anti-corrosion performance

Cold Galvanizing ROVAL Method
ROVAL has been certified to have equivalent anti-corrosion performance as hot-dip galvanizing by the Council for Construction Technology Review and Certification in Japan.
This proved to have the same anticorrosion performance as HDZ55, which is the highest grade of hot dip galvanizing in any of the following cases.
(1) Paint ROVAL 80 μm or more on the blasted steel surface.
(2) Paint ROVAL 40 μm or more on the hot-dip galvanized surface.
Accelerated corrosion test results
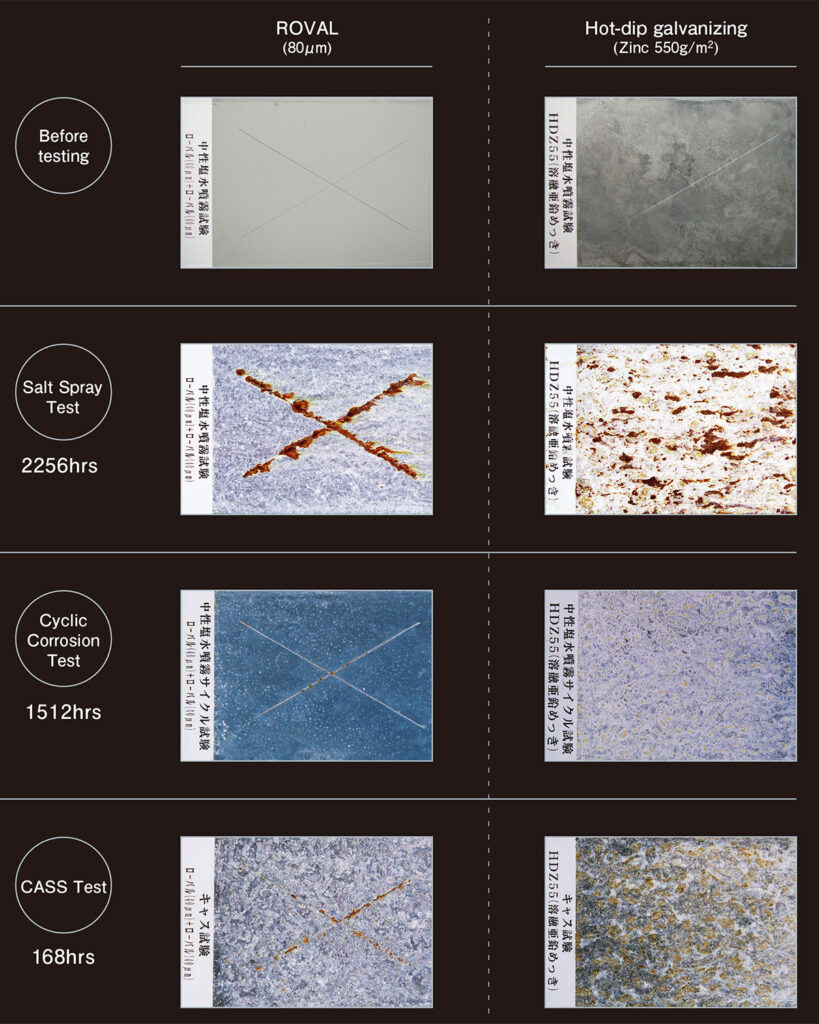
Anti-corrosion performance of ROVAL is equivalent to the highest grade of hot-dip galvanizing available in Japan.
In order to compare the anti-corrosion performance of ROVAL with those from the hot-dip galvanizing process, corrosion accelerating tests were conducted by the Japan Paint Inspection and Testing Association in accordance with Japanese standard “JIS H8502-1999”.
The results and pictures from the test show that ROVAL has an equivalent anti-corrosion performance to hot-dip galvanizing.
ROVAL has been certified to have equivalent anti-corrosion performance as hot-dip galvanizing by the Council for Construction Technology Review and Certification in Japan.


<Salt Spray Test>
Accelerated corrosion testing by spraying salt water
<Cyclic Corrosion Test>
Accelerated corrosion testing involving cyclic exposure to salt fog, dry and wet conditions
<CASS Test>
Copper accelerated acetic acid salt spray test
ISO12944 : 2018 Paints and varnishes — Corrosion protection of steel structures by protective paint systems
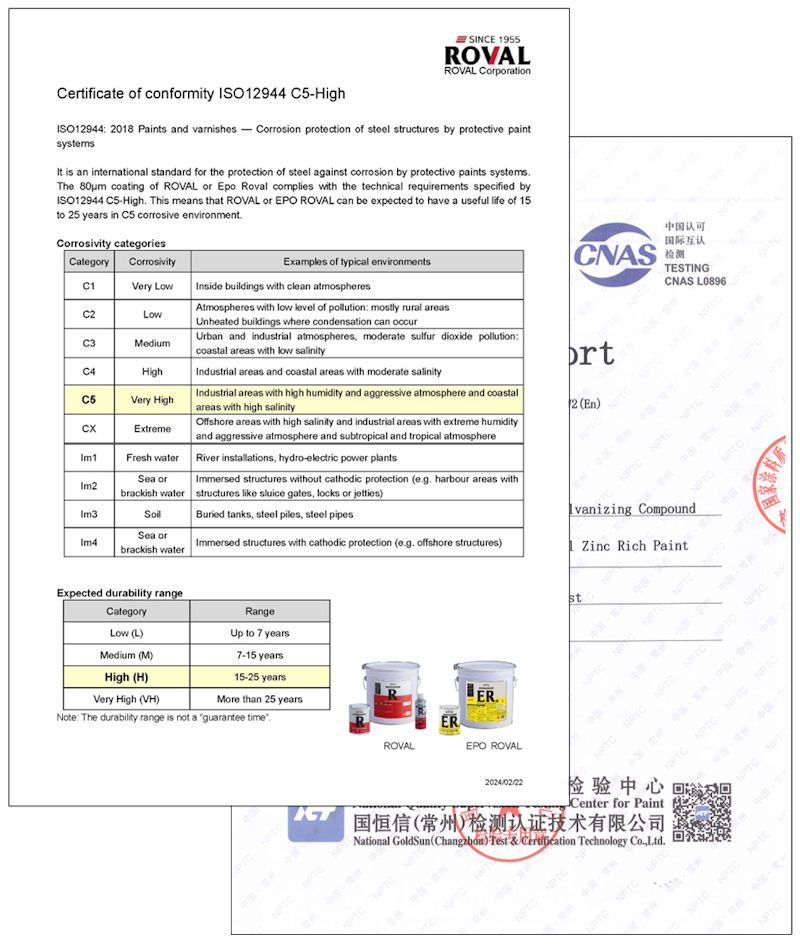
ISO12944 is an international standard for the protection of steel against corrosion by protective paints systems. The 80 μm coating of ROVAL or EPO ROVAL complies with the technical requirements specified by ISO12944 C5-High.
This means that ROVAL or EPO ROVAL can be expected to have a durability life of 15 to 25 years in C5 corrosive environment.
>> Download Certificate
Corrosivity categories
| Category | Corrosivity |
| C1 | Very Low |
| C2 | Low |
| C3 | Medium |
| C4 | High |
| C5 | Very High |
| CX | Extreme |
| Im1 | Fresh water |
| Im2 | Sea or brackish water |
| Im3 | Soil |
| Im4 | Sea or brackish water with cathodic protection |
Expected durability range
| Category | Range |
| Low (L) | Up to 7 years |
| Medium (M) | 7-15 years |
| High (H) | 15-25 years |
| Very High (VH) | More than 25 years |
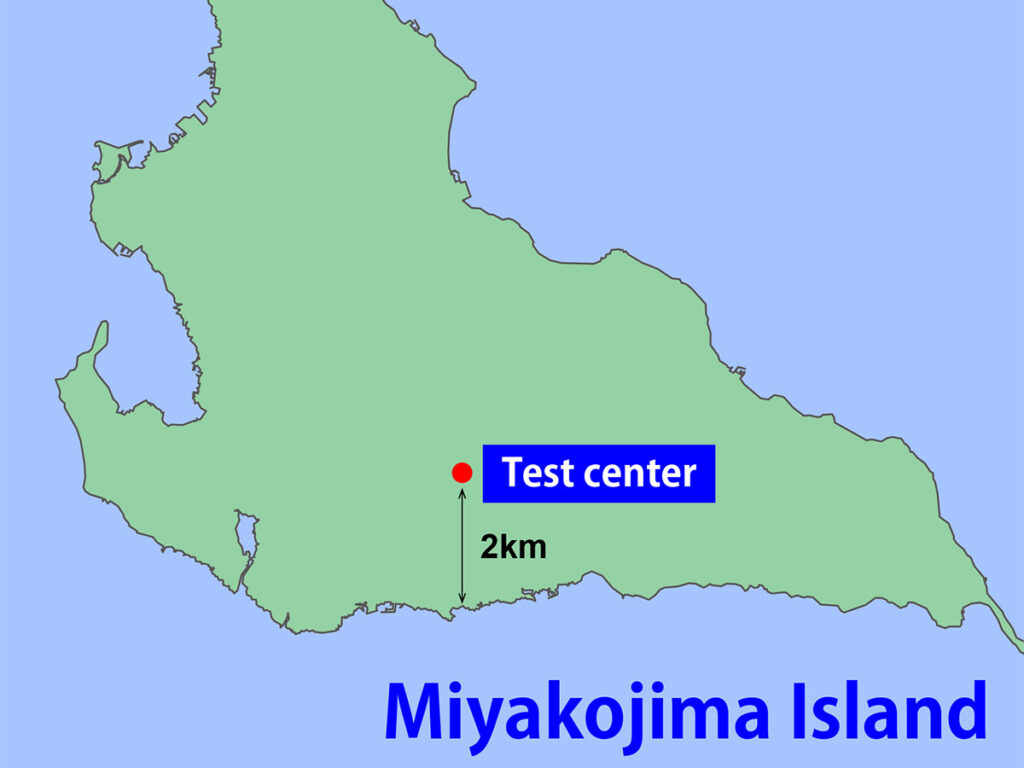
15 year exposure test result in C5 corrosive environment
We conducted an exposure test on Miyakojima Island, where the corrosivity category is the C5 environment. Rust occurred on the entire surface of general paint in 3 years, but ROVAL did not show rust even after 15 years. This is the data that proves compliance with ISO12944 C5-High.
| ROVAL Cold Galvanizing Compound | |||
 |  |  |  |
| Before exposure | 3 years | 10 years | 15 years |
 |  | – – – – – | – – – – – |
| Anticvorrosive paint + Phtalic topcoat |


>> Download Exposure test result
Exposure test results in Miyakojima Island Testing Center
(Comparison with other companies’ painting systems)

To compare the anti-corrosion effect, we conducted exposure tests on Miyakojima Island.
Miyakojima Island is located in the southern area of Japan, at roughly the same latitude as Florida, U.S.A. The island is surrounded by a lot of deterioration factors such as high temperature, high humidity, strong sunshine, and a salt-rich atmosphere.
In such a harsh environment, test pieces of JIS anti-corrosion paint + phthalic topcoat generate rust in one year, and in three years, the rust spreads over the entire surface.
Using Epoxy paint top-coated with a urethane, rust showed up around the cross-cut area.
On the other hand, ROVAL no rusting occurred even after 3 years.
These results and pictures prove that ROVAL protects steel surfaces from rust creepage with its electrochemical reaction, whereas other paints allowed the rust to spread from the cross-cut areas.
20 year exposure test result in a general environment
[ Objective ]
Verify the anti-corrosion performance of ROVAL and Hot-dip galvanizing by an atmospheric exposure.
[ Location ]
Japan Paint Inspection and Testing Association West Branch (JPIA)
| ROVAL (80μm) | Hot-dip galvanizing (550g/m2) | |
| Before exposure |  |  |
| After 5 years |  |  |
| After 10 years |  |  |
| After 15 years |  |  |
| After 20 years |  |  |
[ Result ]
Evaluation after 20 year atmospheric exposure.
ROVAL (Film thickness: 80µm)
— Color change of the film was seen but stayed without rust for 20 years.
Hot-dip galvanizing (HDZ55: 550g/m2)
— Rust occurred on gray zone, which is probably due to corrosion of ferrous of zinc-ferrous alloy layer. However, the rust was only on its surface and not heavy.
Mechanisms of Anti-corrosion
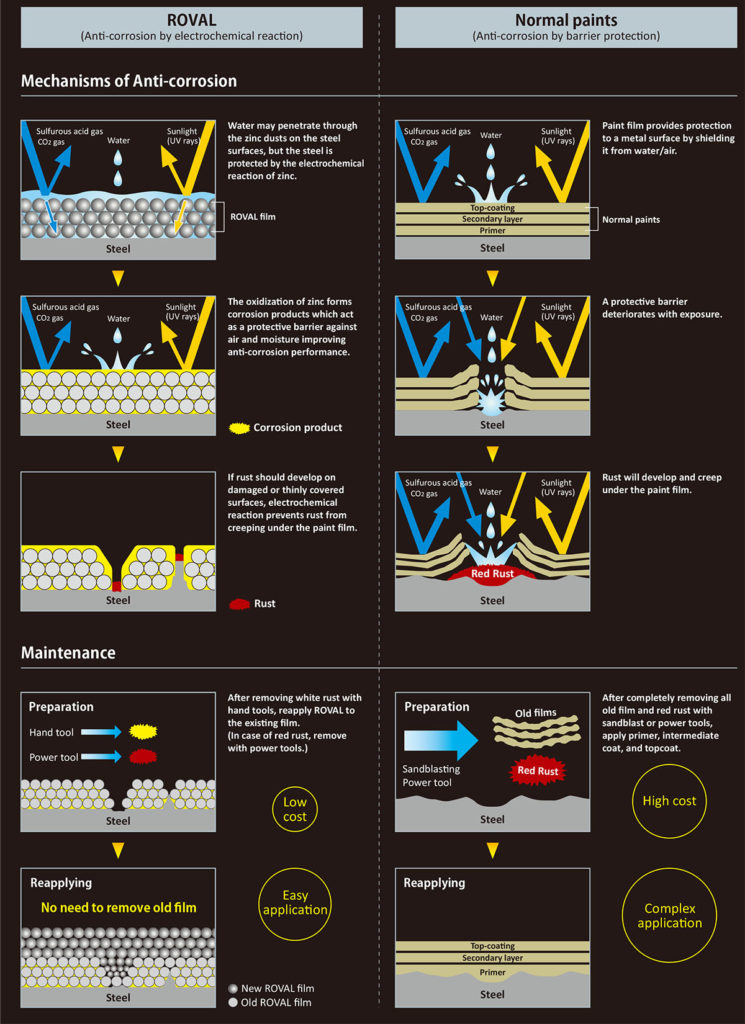
Let me explain the difference in anti-corrosion mechanisms between ROVAL and conventional paints. Conventional paints protect steel by shielding it from water and air, but ROVAL’s protection is based on an electrochemical reaction.
A conventional paint film acts as a surface barrier to water and air. This is called “anti-corrosion by barrier protection”. But once the film is damaged or has deteriorated, rust develops in that area and begins to spread underneath the film.
ROVAL protects steel by the mechanism of electrochemical reaction, employing the self-sacrificing property of zinc. Simply put, keeping the iron and zinc in close contact, only the zinc side will rust and not the iron. Although water and air penetrate through the zinc particles to the steel surface, the electrochemical reaction of zinc prevents further development of rust.
Even when a ROVAL film is damaged, rust will not spread due to the presence of the surrounding zinc particles.